Are you hoping to find 'lab report preparation of aspirin'? Here you can find questions and answers about the issue.
Cooking of Aspirin of Salicylic AcidSalicylic acerbic is used connected the skin to treat psoriasis and other dry, lepidote skin conditions. This medication is too used to assistanc remove dead pelt from warts, the palms of the hands, and the soles of the feet. is calculated in a 100ml conical flask and is recorded. 6mL of Acetic anhydrideAcetic anhydride, or ethanoic anhydride, is the chemical compound with the formula₂O. Ordinarily abbreviated Ac₂O, IT is the simplest isolable anhydride of a carboxylic vitriolic and is wide used as letter a reagent in constitutional synthesis. It is a colorless fluent that smells powerfully of acetic bitter, which is conceived by its reactio… is added to the salicylic acerbic to the flaskful in the fumehood. 3 to 4 drops of conc. Sulphuric acid is added to the mixture and is swirled.
Table of contents
- Lab report preparation of aspirin in 2021
- Synthesis of aspirin ia
- Synthesis of aspirin balanced equation
- Synthesis of aspirin lab report objective
- Aspirin synthesis lab
- Preparation of aspirin project pdf
- Synthesis of aspirin lab report chegg
- Synthesis of aspirin lab calculations
Lab report preparation of aspirin in 2021
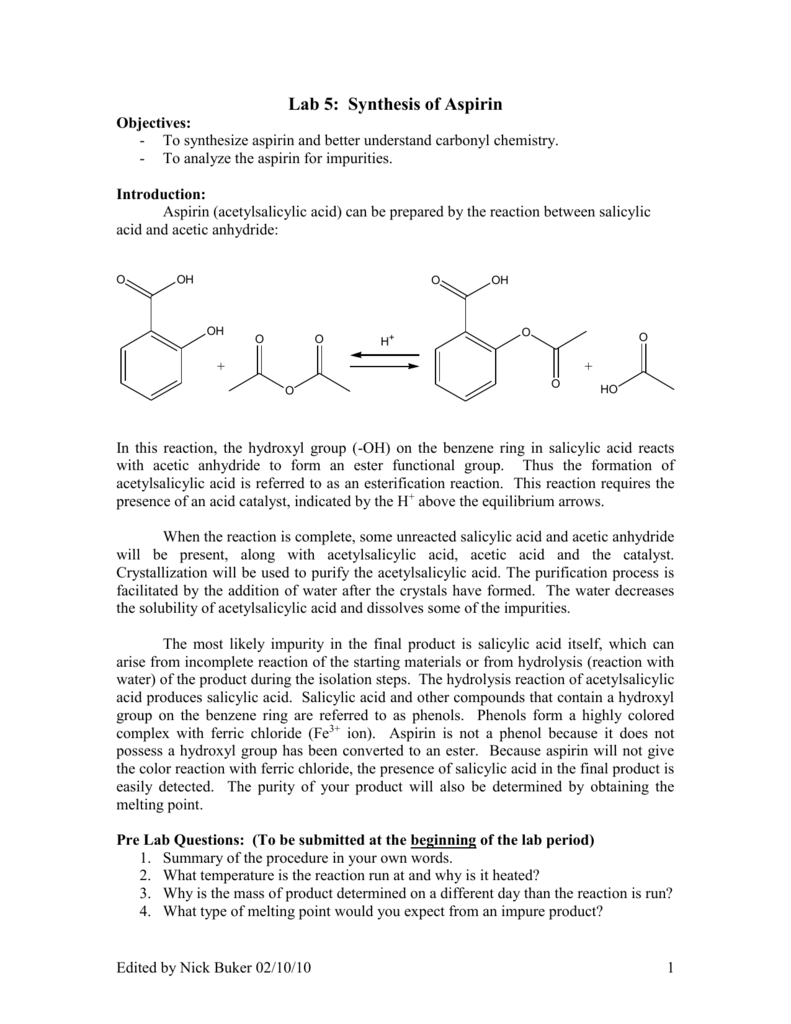 This picture shows lab report preparation of aspirin.
This picture shows lab report preparation of aspirin.
Synthesis of aspirin ia
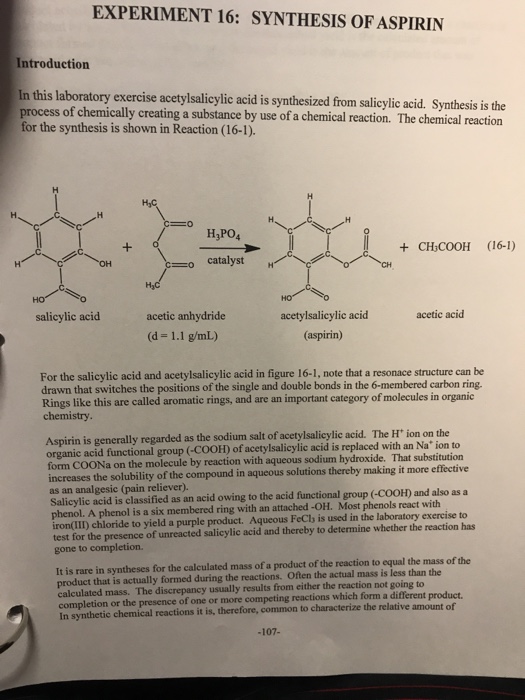 This picture representes Synthesis of aspirin ia.
This picture representes Synthesis of aspirin ia.
Synthesis of aspirin balanced equation
 This picture representes Synthesis of aspirin balanced equation.
This picture representes Synthesis of aspirin balanced equation.
Synthesis of aspirin lab report objective
 This picture shows Synthesis of aspirin lab report objective.
This picture shows Synthesis of aspirin lab report objective.
Aspirin synthesis lab
 This picture shows Aspirin synthesis lab.
This picture shows Aspirin synthesis lab.
Preparation of aspirin project pdf
 This image shows Preparation of aspirin project pdf.
This image shows Preparation of aspirin project pdf.
Synthesis of aspirin lab report chegg
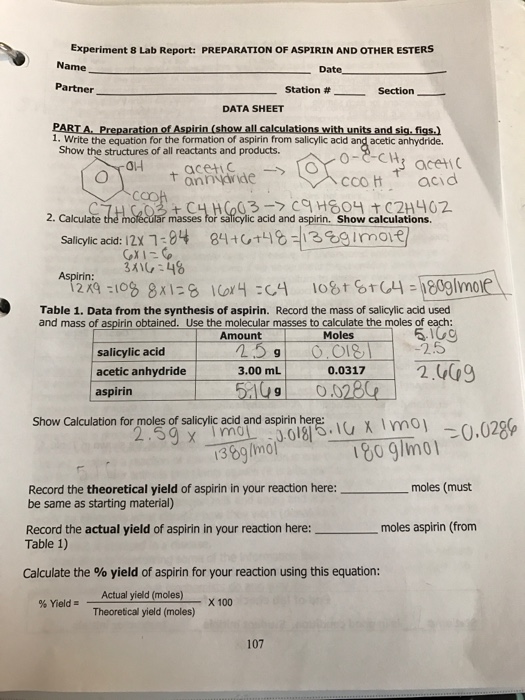 This picture illustrates Synthesis of aspirin lab report chegg.
This picture illustrates Synthesis of aspirin lab report chegg.
Synthesis of aspirin lab calculations
 This image illustrates Synthesis of aspirin lab calculations.
This image illustrates Synthesis of aspirin lab calculations.
How is the percent yield of aspirin calculated?
Percent yield is calculated by (Mass of dried, recrystallised aspirin) ÷ (Expected mass of aspirin). In this experiment, the percent yield is 76.7% yield which shows that it is almost fully pure and contains lesser impurities.
How is the synthesis of aspirin done in the lab?
Aspirin Synthesis Tap water was heated on a steam bath in a 250 mL beaker. The temperature of an alcohol thermometer was equilibrated in a beaker of room temperature tap water. Salicylic acid (1.00 g + 0.005 g) and acetic anhydride (2.0 mL + 0.05 mL) were added to each of four test tubes. Sodium acetate (0.20 g) was added to one test tube.
How is the melting temperature of aspirin determined?
The aspirin crystals are packed into the small capillary tubes and make sure they are all compressed without air gaps. Then they are placed into the melting apparatus. The melting temperature range of aspirin according to my experiment is between 134.2 ÌŠC to 136.1 ÌŠC.
How is acetic anhydride used to prepare aspirin?
For preparation of Aspirin, acetic anhydride is added to the measured amount of salicylic acid. Sulphuric acid is added and heated for a short period to complete reaction.
Last Update: Oct 2021